Get Healthy!
- Posted October 10, 2025
FDA Approves At-Home Version of Lasix for Heart Failure Care
A new at-home version of a common heart failure drug could make treatment easier for millions of Americans.
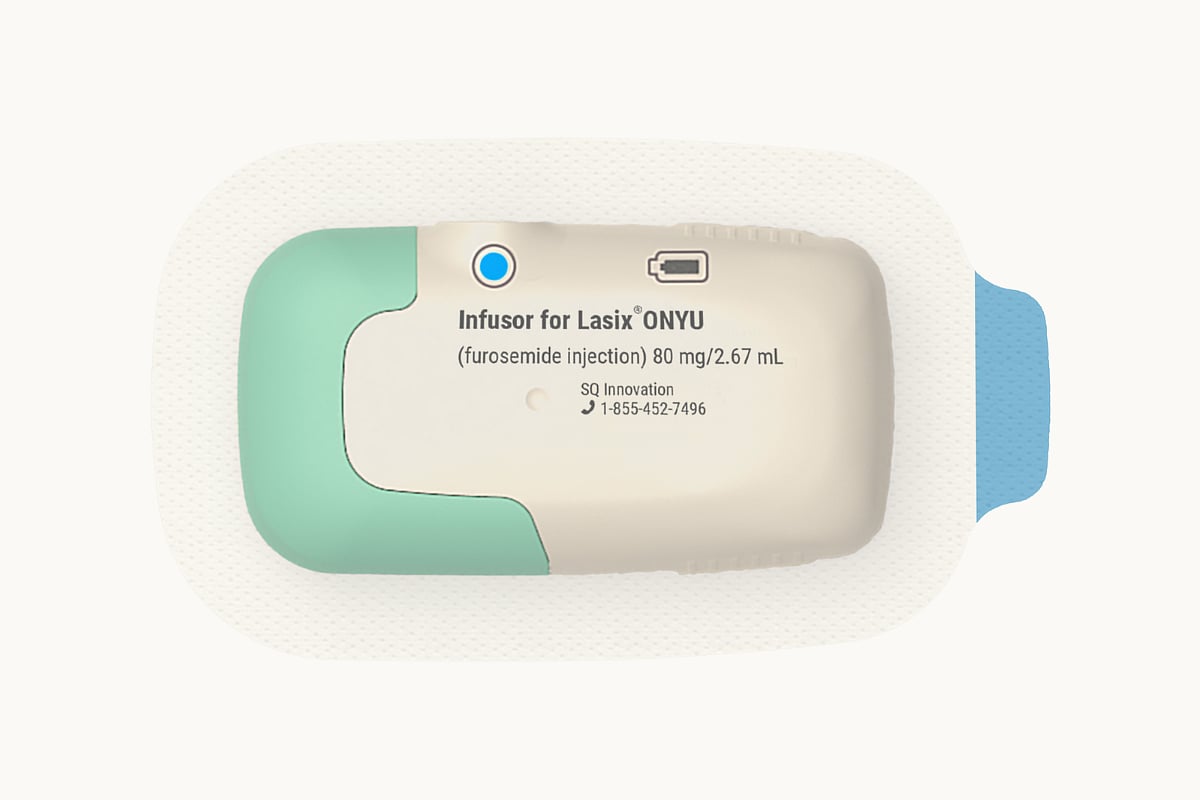
The U.S. Food and Drug Administration (FDA) has approved Lasix ONYU (furosemide injection), a new drug-device combination developed by SQ Innovation, Inc., for treating edema caused by chronic heart failure.
The approval will allow certain patients to receive the treatment at home through subcutaneous (under-the-skin) infusion, rather than in a hospital.
"Lasix ONYU has the potential to be transformative in the care of patients experiencing worsening heart failure due to fluid overload," Dr. Pieter Muntendam, president and CEO of SQ Innovation, said in a news release.
"Treating selected patients at home offers important benefits to patients, health systems and payers," he added, noting that the company expects to launch Lasix ONYU with leading health systems by year’s end.
About 6.7 million Americans live with heart failure, and that number could rise to 8.7 million by 2030, the company said. Heart failure is also a leading cause of hospitalization for people over 65, with 1.2 million hospital stays each year.
Lasix ONYU delivers the same medication found in traditional Lasix IV treatments, but through a small, wearable device.
The system includes a reusable unit that lasts for 48 treatments and a single-use component that is discarded after each session.
The company said this two-part design lowers production costs and could make the therapy more affordable.
In a clinical study, Lasix ONYU showed 112% bioavailability compared to IV Lasix, meaning the body absorbed it fully. It also produced similar results in terms of urine output (115%) and sodium loss (117%), showing that at-home use may be as effective as hospital-based treatment.
"Heart failure is the most common serious medical condition in the U.S. and affects about 1 in 4 Americans during their lifetime," said Dr. Javed Butler, professor of medicine at the University of Mississippi and president of the Baylor Scott & White Research Institute in Dallas.
"The number of patients affected is expected to double over the next 20 years and we currently already often lack adequate resources to take care of the 6.7 million patients affected presently — there are not enough beds, clinicians and funds," he added in a news release.
Others also praised the innovation.
"Decongestion through use of IV diuretics has been the cornerstone of treatment for reducing edema and hypervolemia in heart failure patients for over five decades," said S. Craig Thomas, past president of the American Association of Heart Failure Nurses.
"The availability of accessible, affordable and novel options that do not require the presence of a health care professional allows for transformative new clinical care-delivery," he added. "This means patients who now would typically need to be hospitalized for several days of IV treatment can instead remain home, supported by periodic or remote monitoring."
He said the significance of this shift away from inpatient care cannot be overstated.
Lasix ONYU will be distributed through leading pharmaceutical distributors starting this quarter, making it available at certain hospitals and retail pharmacies nationwide.
More information
Learn more about the Lasix ONYU.
SOURCE: SQ Innovation, news release, Oct. 8, 2025







